Chromatography of Biomolecules
As a simple demonstration of the chromatographic separation of plant pigments I followed this procedure:
- a leaf sample (borrowed from one of my jalapeno plants) was ground up with a small volume of acetone to create the crude extract.


- As much of the liquid as possible was transferred to an eppendorf tube and then spun down at high speed (13k rpm) in a microfuge to sediment out any particulates.
- The supernatant was then transferred to a plastic test tube and placed on a heating block at approximately 70degC to evaporate the acetone. (this step took several hours to complete)


- The solids remaining in the tube were then resuspended in hexane.
- Meanwhile, a separation column was created using a 10ml BD syringe, a small plug of cotton wool and a couple of grams of aluminium oxide. (This is the stationary phase of the column.)
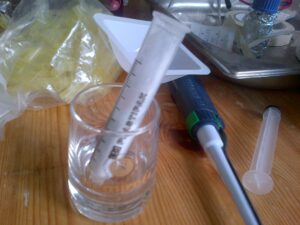
- The column is first wetted with a few mLs of hexane. The column should be saturated in hexane before application of the sample.
- The sample from step 4 was then added to the top of the column with a pasteur pipette and a small amount of pressure applied using the plunger of the syringe.
- Initially clear hexane flows through the column followed by a distinctive yellow fraction (most probably carotenoid pigments), which was caught in another test tube. [While this occurs the green chlorophyll pigments have a higher affinity for the aluminium oxide solid phase than the hexane mobile phase and remain bound to the matrix of the column.]

- Once all of the yellow phase has been removed a few mLs of acetone were then added to the top of the column. After a small amount of clear mobile phase left the column it was followed by a distinctive green phase. [this constitutes the chlorophylls which have a higher affinity to the acetone mobile phase than the stationary aluminium oxide phase and so are eluted from the column]
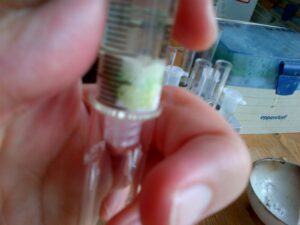

The end result is the separation of these pigments from a single sample, and to demonstrate some basic principles of chromatography.

Much nicer photo of the separated pigments taken by tolland. Right hand red one is an acetone extract from red pepper.
What’s next? : Analysis of other pigmented things. Chromatographic separation of other biomolecules. How about these things? :